Continental Nox Sensor Manual
Has anyone done any research on the Nox sensors from Continental? In looking around the net, Continental is the supplier. There are multiple users of this type sensor, including GM. All of them start out with 5WK9- and then for the 35d 6699C (5WK9-6699C). We pay north of $600, the GM version is 5WK9-6631C available new for $234.95 as a set on ebay. What I'm wondering is this: Is it possible that the only difference in the sensors is the connector?
Navfac design manual. Sep 1, 1986 - This is an inventory of all changes made to this design manual. Added NAVFAC DM's and P-Pubs to Reference list. Alexandria, Virginia APPROVED FOR PUBLIC RELEASE. Foundations &. Earth Structures. DESIGN MANUAL 7.02. REVALIDATED BY CHANGE 1. Publications/Guidance Documents. Q: How do I locate a NAVFAC design manual? A: See Q: What technical policies, criteria or guidance.
Kind of like O2 sensors- universal type if you want to solder/heat shrink or OEM connector for easy install. If they are essentially the same, all we would need is the GM connector and pigtail to adapt to the BMW harness. Does anyone have thoughts? I have looked at this a little bit. And yes, it has been suggested by lpcapital If you need the part, BMW of Bridgewater still sells older releases of the sensors for $220. This part number works for both rear and front sensor (they are same essentially, one with slightly longer cable):6 Upstream NOx sensor in my vehicle threw fault at around 90K miles, I replaced it.
Now, downstream is throwing the same code. Just ordered the above sensor while in stock. Hopefully good for another 50K miles form here on. These sensors are essentially a maintenance item, expected to last about 50K miles.
Seems to be so in my case. Both replaced at 49K under warranty. Upstream failed at 90K, downstream failed at 108K.
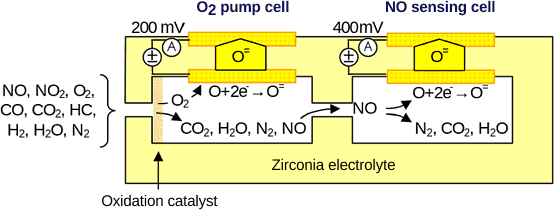
Abstract: Automotive NOx sensors are primarily of the amperometric type, with two or three electrochemical cells in adjacent chambers. The first cell electrochemically pumps O 2 out of the sample so it does not interfere with the NOx measurement in the second cell. Commercial sensors, available from several suppliers, are used for the control of NOx adsorber and SCR aftertreatment. NH 3 sensors have been also developed for use in SCR systems. NOx Sensor Applications The development of exhaust gas NOx sensors started in the 1990s. Commercial sensors were first introduced in the early 2000s on lean-burn, stratified charge gasoline passenger cars with, followed by diesel cars with NOx adsorbers and light- and heavy-duty diesel engines with aftertreatment. The first generation of NOx sensors was developed by NTK, also known as NGK/NTK or NGK Spark Plug (not to be confused with NGK Ceramics) in Japan, and was first used in 2001 in the Volkswagen Lupo 1.4 FSI.
Eventually, all stratified charge gasoline engines in the Volkswagen Group (1.4, 1.6 and 2.0 L) were equipped with NOx sensors. Other OEMs, including Daimler and BMW, also put large numbers of gasoline engines with charge stratification onto the roads. After a few years, however, the use of stratified charge engines and the associated market for NOx sensors started to decline, due to the lower than expected CO 2 emission benefits and the high cost of NOx adsorber aftertreatment. Volkswagen bid farewell to stratified charge engines in 2006, and BMW followed suit five years later. Only Daimler has continued to use spray-guided stratified charging in their M270/M274 engine family.
Another area of NOx sensor application has opened with the introduction of NOx adsorber catalysts on light-duty diesel engines. Some of the first applications included the Toyota system, launched in 2003, and the diesel engine Renault Espace model. The technology was widely adopted on diesel cars—primarily in Europe, but also in the US and other markets—including models from Volkswagen, BMW, and Daimler. These vehicles were typically equipped with a NOx sensor after the NOx storage catalytic converter.
The most recent area of NOx sensor application are urea-SCR systems for light- and heavy-duty diesel engines. To satisfy various OBD (on-board diagnostics) requirements, SCR systems typically use a NOx sensor downstream of the SCR catalyst. If excessive NOx or ammonia concentrations exist at the SCR outlet, an OBD malfunction will be triggered, as NOx sensors are sensitive to both gases. Depending on the strategy, another NOx sensor may be installed in front of the SCR catalytic converter. If two sensors are installed, the conversion rate of the SCR catalytic converter can be easily determined. The most common in-situ NOx measurement technology relies on yttrium-stabilized ZrO 2 (YSZ) electrochemical sensors 984, similar in construction and operating principle to broadband oxygen sensors. Commercial sensors are available from Continental/NGK 3737 and Bosch 3740, while others such as Denso have sensor development programs 3739 3738.
The YSZ sensors are discussed in detail in the following sections. The two final sections of this article cover, respectively, new NOx sensor developments and ammonia sensors. The latter technology is based on the same YSZ electrochemical system, has been commercialized in some SCR applications, but its use remains limited. Principle of Operation Overview Commercial NOx sensors for automotive applications are primarily YSZ electrochemical sensors of the amperometric type. Figure 1 illustrates the basic operating principle.
The sensor uses two or three electrochemical cells in adjacent chambers. The first cell electrochemically pumps O 2 out of the sample so it does not interfere with the NOx measurement in the second cell.
The need to remove O 2 allows this type of NOx sensor to serve a dual purpose; it can also detect exhaust O 2 level. Schematic representation of an amperometric NOx sensor The O 2 in the first cell is reduced and the resulting O ions are pumped through the zirconia electrolyte by applying a bias of approximately -200 mV to -400 mV. The pumping current is proportional to the O 2 concentration. The remaining gases diffuse into the second cell where a reducing catalyst causes NOx to decompose into N 2 and O 2.
As with the first cell, a bias of -400 mV applied to the electrode dissociates the resulting O 2 which is then pumped out of the cell; the pumping current of the second cell is proportional to the amount of oxygen from the NOx decomposition. An additional electrochemical cell can be used as a Nernstian lambda sensor to help control the NOx sensing cell 3741.
All HC and CO in the exhaust gas should be oxidized before the NOx sensing cell to avoid interference. Also, any NO 2 in the sample should be converted to NO prior to NOx sensing to ensure the sensor output is proportional to the amount of NOx. Solid Zirconia Electrolyte A number of zirconia formulations doped with metal oxides have been investigated for use in oxygen (λ, lambda), as well as NOx sensors. Materials that have been tested include Fe 2O 3, Co 3O 4, NiO, CuO, ZnO, CeO 2, La 2O 3, Y 2O 3, as well as mixtures of zeolites, aluminum and silicates 3892 3894 3893.
Several chemical elements were also selected as potential electrode materials, including platinum, rhodium and palladium. The system that has been most widely adopted and used in almost all commercial NOx and lambda sensors is based on solid state yttrium-stabilized zirconia electrolyte (the same material that was used in the Nernst lamp). A key property of the YSZ ceramics is its high conductivity for O 2 ions at elevated temperatures. The stabilization with yttrium has two benefits: (1) it impedes ZrO 2 phase transformation, which increases the mechanical strength of the material, and (2) it enhances the oxygen ion conductivity of zirconia.
Zirconium oxide ceramics can have one of three crystalline phases, depending on the temperature 3891:. Monoclinic crystal structure at room temperatures. Tetragonal crystal structure from 1,170°C.
Cubic crystal structure from 2,370°C The cubic crystal structure displays a particularly regular arrangement of elements, and is characterized by high oxygen ion conductivity. Through the addition of metal oxides, the high temperature crystal structures can remain stable at lower temperatures. By adding sufficient quantities of yttrium oxide (Y 2O 3) in a sintering process at approximately 1,000°C, it is possible to cubically stabilize zirconium oxide. If the yttrium oxide quantities are too low, mixed crystals form, consisting of the monoclinic and cubic phase. These partially stabilized zirconium oxide (PSZ) materials feature a pronounced resistance to thermal fluctuations.
Two types of YSZ ceramics, 4YSZ and 8YSZ, are the basis of almost all lambda and nitrogen oxide sensors. These designations indicate the level of doping with yttrium oxide, as follows:. 4YSZ—partially stabilized ZrO 2 doped with 4 mol% of Y 2O 3. 8YSZ—fully stabilized ZrO 2 doped with 8 mol% of Y 2O 3 When zirconia is stabilized with yttrium oxide, the Y 3+ ions replace Zr 4+ in the atomic lattice. This way, two Y 3+ ions generate one oxygen gap. These gaps are utilized for the transport of oxygen. The maximum oxygen ion conductivity is observed within the temperature range from 800°C to 1,200°C.
Continental Smart Nox Sensor Manual
Unfortunately, at these temperatures a separation also occurs into Y-lean and Y-rich areas. This process is irreversible and results in a severe reduction in oxygen conductivity. At 950°C, O 2 conductivity can be reduced by as much as 40% after 2,500 hours 3891. This is the reason why lambda and NOx probes may not be subjected to temperatures above approximately 930°C. Nitrogen oxide sensors by Continental, for example, are operated at 800°C 2827.
Oxygen Pump Cells If a dividing wall made of YSZ ceramics is placed between two chambers with different oxygen partial pressure, nothing will happen at room temperature. However, when the temperature of the ceramic wall is increased to approximately 600°C, oxygen ions can move through the gaps in the crystal lattice. An alignment takes place, where the chamber with the higher partial pressure pushes oxygen ions through the wall to the chamber with the lower pressure. If both surfaces of the dividing wall are fitted with an electrode, it is possible to verify the movement of ions through voltage measurement. And this is precisely what happens in the binary (switching) lambda sensor. The reduction of oxygen to O 2- that occurs in the chamber of a higher O 2 pressure is described by Equation (1): (1)O 2 + 4e- = 2O 2- and the sensor voltage is given by the Nernst equation: (2)U s = (RT/4F) ln(p ref / p exh) where: U s - sensor signal, V T - temperature, K p - partial pressure of oxygen R - gas constant = 8.314 J/mol F - Faraday constant = 96,485 sA/mol The diagram in Figure 2 presents the chamber with high oxygen partial pressure as the blue-colored area, and the chamber with low oxygen partial pressure as the gray area. If the brown-colored ceramic is heated to 600°C, the micro-porous platinum electrodes presented in yellow will generate approximately 1V.
03c907807d
Schematic of a solid zirconia electrolyte cell Passive Cells. The chamber with the high partial pressure of oxygen is the reference air duct. Rich exhaust gas (λ 1), the oxygen partial pressure difference relative to the reference air is low and a signal of only 0.1V or less is measured. At λ = 1, the signal voltage is approximately 0.4-0.5V, depending on the manufacturer and probe model. The voltage-lambda characteristic is almost stepwise, allowing the sensor to distinguish between two lambda values—rich and lean—hence the term “binary” lambda sensor. In such operation—representative of a binary lambda probe—the generated voltage correlates with the drop in oxygen partial pressure. The passive YSZ ceramics cell is also called the potentiometric or Nernst cell.

Active Cells. It is also possible to actively operate the probes, as is the case in broadband (linear) oxygen sensors and in the amperometric cells in NOx sensors. In active operation, no voltage is picked up on the electrodes, but rather the electrodes are connected to a power source. In such active cells—referred to as “pump cells”—it is possible to “pump” oxygen ions from the oxygen-lean to the oxygen-rich side by reversing the polarity. The pumping current provides a measure of oxygen concentration.
The current-lambda characteristic is linear, which makes it possible to measure O 2 concentrations at various air-to-fuel ratios. NOx sensors include at least two oxygen pump cells (Figure 1)—one to remove excess oxygen from the exhaust gas, and another to measure the concentration of oxygen released from the decomposition of NOx. Acknowledgements We appreciate the assistance from Dirk Bleicker of Carit Automotive GmbH, who provided information and images on dosimeter-based NOx sensors.